Abstract
Allogeneic hematopoietic stem cell transplant (alloHCT) remains the only potentially curative treatment for patients with myelodysplastic syndrome (MDS). However, this treatment is associated with significant risk of transplant-related mortality/morbidity such as graft-versus-host disease, infections, and regimen-related toxicities. Since there has been no "randomized" trial comparing between patients undergoing or not undergoing transplantation, the relative benefit of this treatment, particularly in elderly patients, is largely unknown. Retrospective comparative studies are significantly limited by the inherent selection bias of healthier/well-supported patients in the alloHCT group. Therefore, a critical knowledge gap exists regarding the survival outcome of MDS patients who are transplant eligible yet did not undergo alloHCT due to lack of suitable donors or other reasons.
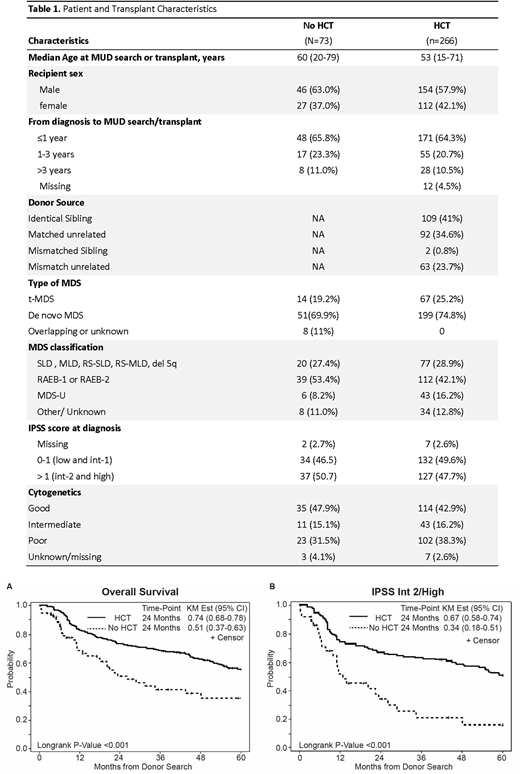
Herein, we retrospectively identified a consecutive case-series of 73 patients with MDS (excluding CMML), who were considered alloHCT candidates, based on initiation of an official donor search from 2005 to 2015, yet did not proceed with alloHCT. Median age at time of donor search was 60 years (range: 20-79) with the majority (63%) being male. Classifications of MDS were single or multi-lineage dysplasia (n=20), excess blast (n=39), MDS unclassified (n=6) or other/unknown classification (n=8). The cohort included 51 de novo MDS and 14 therapy-related MDS (t-MDS). Per International Prognostic Scoring System (IPSS) 29 patients (39.7%) were Intermediate (Int)-1, 14 (19.2%) were Int-2, and 23 (31.5%) were high risk at the time of donor search (Table 1). Reasons for no alloHCT were lack of donor (n=29), persistent/progressive disease (n=9), patient choice (n=13), or infections/complications after initiating the donor search (n=18). Treatments of these patients included chemotherapy (n=14), hypomethylating agents (n=61) and supportive care (n=23). Of the 73 patients, 15 (20.5%) had disease progression to acute leukemia at 1 year. There were 38 deaths with the median OS of 26.2 months (95%CI: 17.3-48.3 months). The 2-year probability of OS was 51% (95%CI: 36.7-62.9%).
We next compared outcomes of these MDS patients who had a donor search without subsequent HCT to a consecutive case-series of MDS patients who underwent alloHCT from matched related and unrelated donors (cord blood and haploidentical transplants were excluded) during the same time period (n=276) at our center (Aldoss et al. Haematologica 2017). Patient demographics and MDS disease characteristics were similar between the two groups (Table 1). Median number of days from HLA typing to HCT were 168. By Kaplan-Meier method, OS (from the time of donor search) was significantly better for the alloHCT group (74% at 2-years) compared with non-HCT group (51% at 2-years), log-rank P<0.001 (Figure 1a). This survival benefit was primarily driven by the subgroup of patients with int-2/high risk IPSS. While the difference in the OS did not reach statistical significance in low/int-1 patients between HCT and non-HCT groups (OS probability at 2-years: 80% vs 68%, respectively, p= 0.182), the 2-year OS was significantly better (p<0.001) in the alloHCT group (67%, n=133) compared with non-HCT group (34%, n=37), when analysis was done in int-2 or high-risk patients. (Figure 1b) In an attempt to further assess the inherent selection bias, we analyzed and compared patients with no available donor (n=29, biologic assignment) with patients who did not receive HCT for other reasons (n=44). No statistically significant difference (p=0.13) was seen in the 2 year-OS (58% vs. 45%).
In conclusion, using a unique cohort of patients who were referred for a donor search, our study in real-world practice demonstrates that transplant eligible MDS patients (at the time of donor search) who do not undergo alloHCT have worse survival outcomes compared to those undergoing transplantation. A prospective biologic assignment study is currently underway by the BMT CTN (#1102) to more definitively determine the impact and relative benefits of alloHCT in patients (≥50 years old) with Int2/high-risk de novo MDS.
Khaled:Alexion: Consultancy, Speakers Bureau; Daiichi: Consultancy; Juno: Other: Travel Funding. Salhotra:Kadmon Corporation, LLC: Consultancy. Ali:Incyte Corporation: Membership on an entity's Board of Directors or advisory committees. Forman:Mustang Therapeutics: Other: Licensing Agreement, Patents & Royalties, Research Funding. Stein:Celgene: Speakers Bureau; Amgen Inc.: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.